
When you’re pregnant, every pill, drop, or supplement feels like a gamble. You don’t want to risk your baby’s health, but you also can’t ignore your own needs. What happens when you need an antidepressant, an asthma inhaler, or even a simple painkiller? The answer isn’t simple - and that’s exactly why safety alerts exist.
Why Medication Safety During Pregnancy Is So Complicated
Most drugs aren’t tested on pregnant women. Not because scientists don’t care, but because ethical rules block it. Clinical trials almost always exclude pregnant people unless the drug is meant to treat a pregnancy-specific condition. That means for the vast majority of medications, we’re flying blind when it comes to real human data. The result? Only 5 to 10% of FDA-approved drugs between 2003 and 2012 had enough human pregnancy safety data to make solid recommendations. Today, nearly 70% of pregnant women take at least one medication. Half take four or more. And 40 to 80% of pregnancies are unplanned. So, many people are already taking drugs before they even know they’re pregnant. This isn’t just a numbers game. It’s a life-and-death balancing act. Untreated depression can lead to preterm birth. Uncontrolled seizures can harm the fetus. High blood pressure without medication can cause stroke or placental damage. But so can some drugs.The Old System Was Broken - Here’s What Changed
Before 2015, the FDA used letters: A, B, C, D, X. It sounded simple. But it wasn’t. Many people thought an “X” meant “dangerous,” and a “B” meant “safe.” That’s not what they meant. A “C” didn’t mean “moderate risk.” It meant “animal studies showed harm, but human data was missing.” That confusion led to dangerous decisions - like stopping life-saving medications out of fear. In 2015, the FDA scrapped the letter system. It replaced it with the Pregnancy and Lactation Labeling Rule (PLLR). Now, drug labels have clear narrative sections: Pregnancy, Lactation, and Females and Males of Reproductive Potential. No more vague letters. Just facts - or the lack of them. But here’s the catch: only 32% of these new labels include actual numbers. Like, “This drug increases the risk of heart defects by 2%.” Most still say things like “potential risk cannot be ruled out.” That’s not helpful when you’re trying to decide whether to keep taking your medication.What Safety Alerts Actually Do
Safety alerts aren’t warnings to avoid all drugs. They’re targeted signals that something new has been found - and it matters. In 2022, the FDA issued 17 pregnancy-related safety alerts. Four were Class I - the most serious. One was for valproate, a seizure and bipolar medication. It raised the risk of neural tube defects from 0.1% to 1-2%. That’s a tenfold increase. Another alert warned against using isotretinoin (Accutane), which causes severe birth defects in 20-35% of exposed pregnancies. These alerts don’t just appear out of nowhere. They come from pregnancy exposure registries. These are voluntary programs where doctors and patients report drug use during pregnancy. The FDA runs 38 of them. Each one tracks women from early pregnancy through delivery and beyond. But here’s the problem: less than 1% of all pregnant women taking medications are enrolled in these registries. That means for every 100 women who take a drug, only one is helping build the data that could protect the next 100. And because data collection is slow, safety alerts often come years after harm is already happening.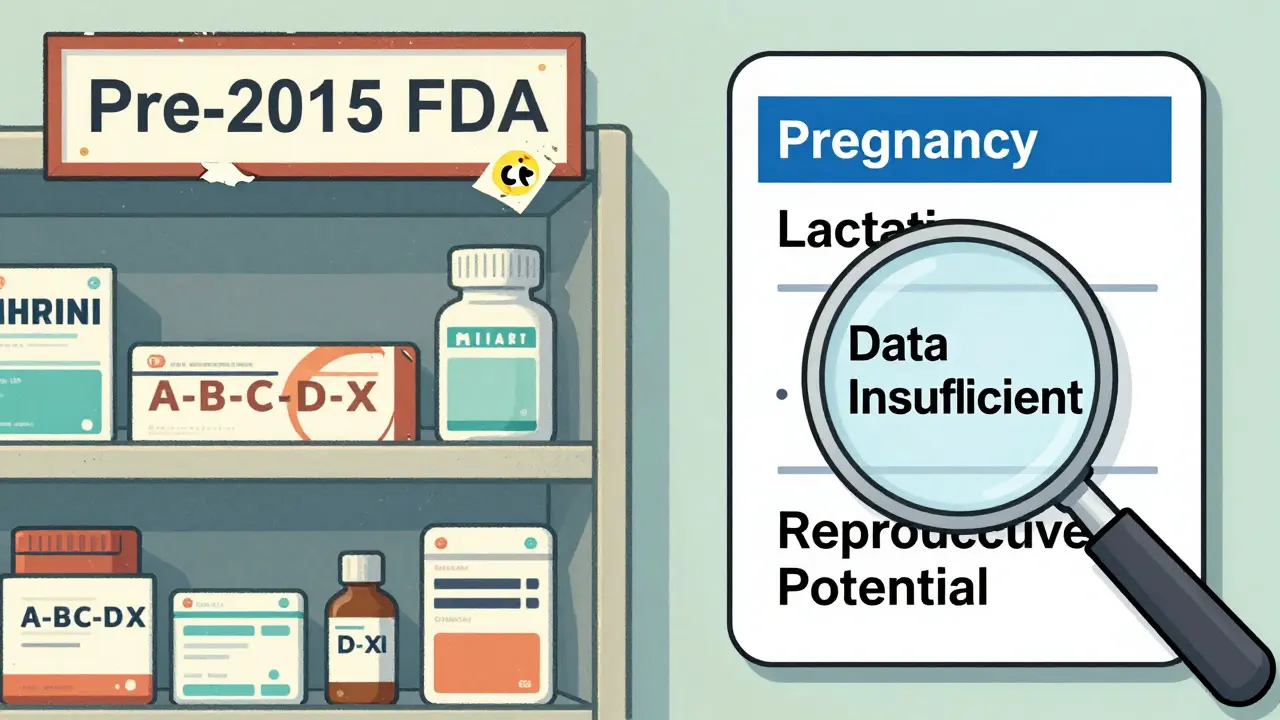
How the U.S. and Europe Compare
The U.S. and Europe both want to protect pregnant people. But they do it differently. The FDA focuses on clear labeling. The European Medicines Agency (EMA) goes further. For high-risk drugs like lenalidomide (used for multiple myeloma), the EMA requires mandatory pregnancy testing, two forms of contraception, and even a signed agreement from the patient before the drug is dispensed. It’s strict. But it works. In Europe, 41% of drug companies failed to meet basic pregnancy monitoring requirements in a 2022 audit. In the U.S., only 22% of eligible companies maintain active pregnancy registries. Neither system is perfect. But Europe’s proactive controls catch more risks early. The U.S. system has one big advantage: it’s easier for patients to understand. The EMA’s rules are buried in complex regulatory documents. Most women never see them.What You Should Do - Step by Step
You don’t need to be a doctor to protect yourself and your baby. Here’s what actually works:- Make a full list of everything you take - prescriptions, over-the-counter pills, vitamins, herbal teas, CBD oils. Don’t leave anything out. Even “natural” can be risky.
- Bring that list to your first prenatal visit. ACOG recommends a full medication review at your first appointment. It takes about 22 minutes. That’s less than your coffee break.
- Check the drug label. Look for the “Pregnancy” section. If it says “data insufficient,” don’t panic. Ask your doctor: “Is there a safer alternative? What happens if I stop?”
- Don’t stop meds without talking to your provider. A 2021 survey found that 29% of women with chronic conditions stopped their meds as soon as they found out they were pregnant. That led to worse outcomes than staying on treatment.
- Take folic acid - 800 mcg daily. It’s one of the few things proven to prevent birth defects. Start before you conceive if possible. Keep taking it until at least 12 weeks.
- Avoid known dangers. Isotretinoin, valproate, methotrexate, and certain antibiotics like tetracycline are off-limits. If you’re on any of these, talk to your doctor before trying to get pregnant.
What’s Being Done to Fix This
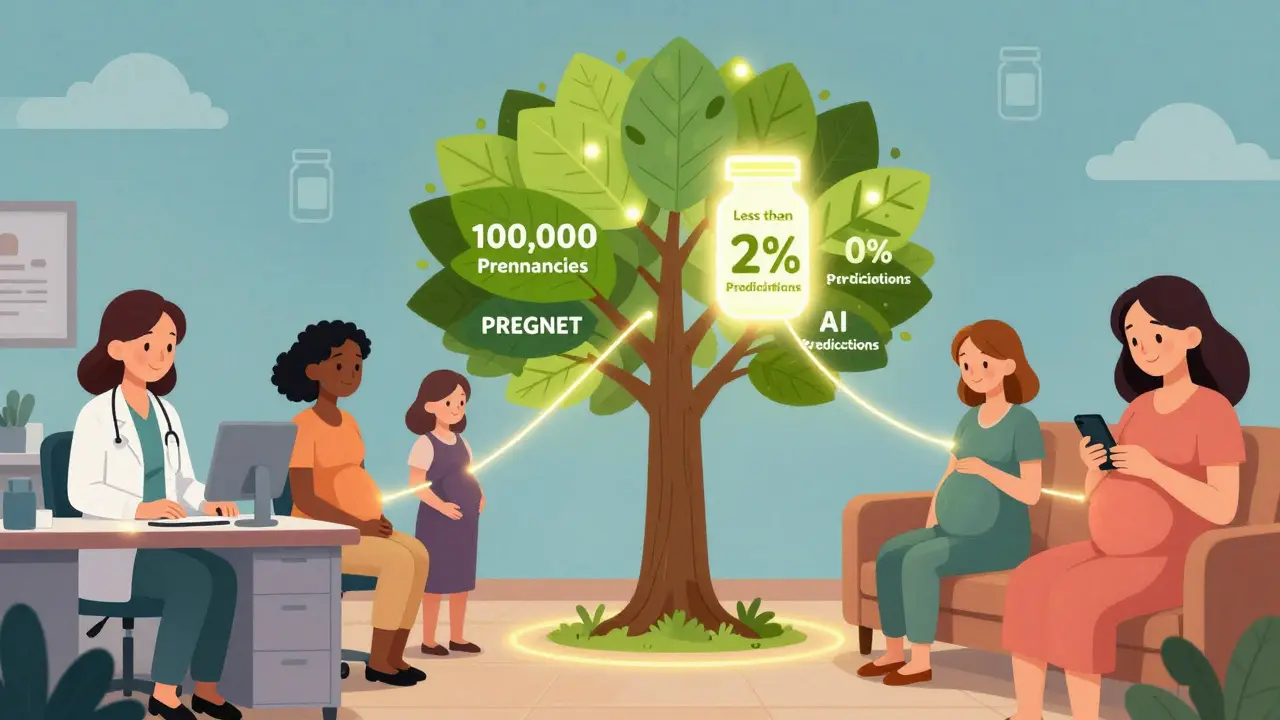
There’s hope. The NIH launched PREGNET in January 2024 - a $25 million project to link 45 medical centers and track 100,000 pregnancies over five years. That’s the biggest effort ever to collect real-world pregnancy drug data. Pharmaceutical companies are building apps to help track medication use during pregnancy. But only 12% of them have real user engagement. Most are just digital brochures. AI is coming. IBM Watson Health predicts that within five years, machine learning models will predict medication risks with 70% accuracy by analyzing millions of anonymized pregnancy records. That could cut the delay between harm and alert from years to months. But none of this matters without funding. The March of Dimes estimates a $312 million annual gap in funding for pregnancy safety monitoring through 2030. Without investment, these systems will keep crumbling.Real Stories Behind the Data
On Reddit’s r/Bump, a woman wrote: “My doctor told me to stop my antidepressant immediately. Now I’m having panic attacks. Why isn’t there clearer guidance?” That’s not rare. A study from Massachusetts General Hospital tracked 12,500 pregnant women. They found that 78% of calls to their safety hotline were about anxiety meds. And in 78% of those cases, the recommendation was to keep taking them - not stop. Another woman on Drugs.com said: “One site said my blood pressure med was safe. Another said it was dangerous. I panicked and stopped. Now my BP is out of control.” Contradictory info is the #1 complaint. That’s why talking to your provider - not Google - is the only reliable path.Final Reality Check
No medication is 100% safe during pregnancy. But no condition is 100% safe to leave untreated, either. The goal isn’t to avoid all drugs. It’s to make informed choices. To know what’s risky. To know what’s necessary. To know that your health matters just as much as your baby’s. If you’re pregnant or planning to be, don’t wait for a safety alert to come out. Don’t rely on a letter on a bottle. Ask questions. Get help. Use the tools that work: medication reconciliation, folic acid, and open conversations with your care team. Because in pregnancy, safety isn’t about perfection. It’s about smart, supported decisions - one pill at a time.Are all prescription drugs dangerous during pregnancy?
No. Many commonly used medications - like certain antibiotics, thyroid drugs, and prenatal vitamins - are considered safe or low-risk when used as directed. The key is knowing which ones are safe for your specific situation. Always consult your provider before starting, stopping, or changing any medication during pregnancy.
Can I trust drug labels for pregnancy safety info?
Drug labels under the FDA’s Pregnancy and Lactation Labeling Rule (PLLR) are the most reliable source, but they’re not perfect. Many still lack specific risk numbers. If the label says “data insufficient,” that means there’s no clear answer - not that it’s definitely dangerous. Always follow up with your doctor for personalized advice.
What should I do if I took a medication before knowing I was pregnant?
Don’t panic. Most medications don’t cause harm in early pregnancy, especially before organ development begins (around week 4-10). Contact your doctor right away. They’ll assess the drug, timing, and dosage. In many cases, no action is needed. If a high-risk drug was taken, your provider may recommend extra monitoring, like a detailed ultrasound.
Is it safe to take over-the-counter painkillers like ibuprofen while pregnant?
Avoid ibuprofen and other NSAIDs after 20 weeks of pregnancy - they can affect fetal kidney function and reduce amniotic fluid. Acetaminophen (Tylenol) is generally considered the safest option for pain or fever during pregnancy, but even that should be used at the lowest effective dose for the shortest time possible.
Why don’t doctors always know if a drug is safe in pregnancy?
Because pregnant women are excluded from most clinical trials, doctors often have to rely on animal studies, small case reports, or registries with limited data. For many drugs, there simply isn’t enough human evidence yet. That’s why safety alerts take years to appear - and why ongoing monitoring through pregnancy registries is so critical.
How can I help improve pregnancy medication safety?
If you’re pregnant and taking a medication, consider joining a pregnancy exposure registry. These are voluntary programs run by the FDA or academic centers. Your participation helps build the data that protects future mothers and babies. Ask your doctor if one exists for your medication - or visit the FDA’s Pregnancy Registry website to find one.






10 Comments
It's ridiculous that we still don't have better data on what's safe during pregnancy. We track every step of a baby's development but act like drugs are some kind of mystery box. The FDA's new labeling system sounds good on paper but if 68% of labels just say 'we don't know,' that's not transparency-it's negligence.
And don't get me started on how doctors panic and tell women to stop antidepressants like it's a switch. You don't just flip off your brain chemistry because you're pregnant. The real danger is the fear, not the medication.
We need mandatory reporting from pharmacies. If you're prescribed a drug during pregnancy, it should auto-enroll you in a registry. No opt-in. No paperwork. Just data. That's how you fix this.
And stop calling it 'risk.' It's not risk. It's uncertainty. There's a difference.
Someone needs to hold these drug companies accountable. They make billions off these meds but won't fund the studies that could save lives. That's not capitalism. That's criminal.
So let me get this straight-we ban isotretinoin because it causes birth defects, but we let women take Tylenol every day like it’s candy? Funny how the ‘safe’ drugs are the ones we’ve been using since the 80s and nobody bothered to test them properly.
Also, folic acid is the only miracle pill we’ve got? That’s it? We’re betting on a vitamin to fix a system built on guesswork?
And why is it always the woman’s job to ‘be careful’? Where’s the male responsibility in this? Men take meds too. Where’s the registry for sperm donors on antidepressants?
Also, the EMA has stricter rules? Cool. So why do we still let every pharmacy in America hand out ibuprofen like it’s gum? Hypocrisy is a drug too.
70% of pregnant women take meds. Half take four or more. And you want me to believe that’s not a public health disaster waiting to happen?
Registries have 1% participation. AI models are five years away. The FDA’s new labels are 68% useless. And you’re telling me this is the best we can do?
It’s not complicated. It’s just ignored.
Stop pretending there’s a solution when the system is designed to fail.
Also, folic acid doesn’t fix everything. It’s a Band-Aid on a gunshot wound.
Just curious-how many of these safety alerts are triggered by actual harm vs. corporate liability? Like, does a drug get flagged because a baby had a defect… or because a lawyer found a registry entry?
Also, if 80% of pregnancies are unplanned, why aren’t we testing all women for meds before they even know they’re pregnant? Like a blood test at the ER when they come in for a missed period?
And why is it always the mom’s job to fix this? Why isn’t there a national database that flags high-risk meds for *all* reproductive-age people, male and female?
Just asking. Not judging. Just… confused.
Oh wow 🤯 so you mean… women should actually talk to their doctors instead of Googling ‘is my Zoloft gonna turn my baby into a lizard’? 🤦♀️
And folic acid? 800 mcg? Whoa. That’s like… science. Who knew?
Also, if you’re on valproate and didn’t know you were pregnant… congrats, you’re now the reason we need a new FDA slogan: ‘We Told You So.’
Also, why are we still using ‘data insufficient’ like it’s a magic spell? It’s not a disclaimer. It’s a failure.
And yes, I’m judging you. You took ibuprofen after 20 weeks. I saw your cart. 😘
It’s not just about medication-it’s about trust. In the U.S., we treat pregnancy like a medical emergency waiting to happen. In India, we often rely on tradition, but even there, the lack of access to proper care leads to silent risks.
What’s missing isn’t just data-it’s communication. A doctor in rural Ohio and a nurse in rural Bihar both face the same problem: no clear answers, no time to explain, no follow-up.
The solution isn’t AI or registries alone. It’s training frontline health workers to ask the right questions. Not ‘Are you taking anything?’ but ‘What are you taking, and why?’
And yes, folic acid matters. But so does clean water, nutrition, and a supportive partner. These aren’t ‘extras.’ They’re part of the equation.
Let’s stop treating pregnancy like a math problem with pills as variables. It’s a human experience. We need systems that honor that.
Also, if you’re on a medication that’s not on the list, ask your doctor. Don’t assume. Don’t panic. Just ask.
so like… if i took ibuprofen before i knew i was pregnant is my baby gonna be fine
also why does every site say something different
my dr said stop my anxiety med but my therapist said dont
now im just crying in the pharmacy aisle
why is this so hard
My wife took Zoloft through all three trimesters. Baby’s now 3. No issues. No seizures. No weird skin. Just a kid who loves dinosaurs.
Meanwhile, her OB kept saying ‘maybe stop’ like it was a recommendation.
Turns out the real risk was the fear.
Also, folic acid is free. Take it.
Also, stop Googling.
Also, your doctor doesn’t know everything.
But they’re still your best shot.
Why are we still talking about pregnancy as if it’s a disease state? You’re not sick. You’re pregnant. The body adapts. The system doesn’t.
Also, the FDA’s labeling change was just rebranding. Same garbage, new font.
And AI predicting risks? Cute. We still don’t have a basic registry that works.
Meanwhile, in Europe, they make you sign a contract before you get a pill. In the U.S., you get it with your coffee.
We’re not protecting mothers. We’re protecting lawyers.
Also, folic acid is not a solution. It’s a distraction.
PLEASE, PLEASE, PLEASE-every single person reading this: write down EVERYTHING you take. Every pill. Every tea. Every gummy. Every topical cream. Every essential oil. Every supplement labeled “natural” or “herbal.”
Bring it to your provider. Don’t say “I think it’s fine.” Don’t say “I forgot.” Don’t say “It’s just a little.”
Write it. Down. On. Paper.
And if your provider doesn’t ask for it? Ask them: “Can we do a full med review?”
And if they say “No, we don’t have time”? Find a new provider.
Because your baby’s life is not a 22-minute afterthought.
And yes, folic acid. 800 mcg. Daily. Before, during, after. No exceptions.
You are not being dramatic. You are being responsible.
And you deserve better than guesswork.