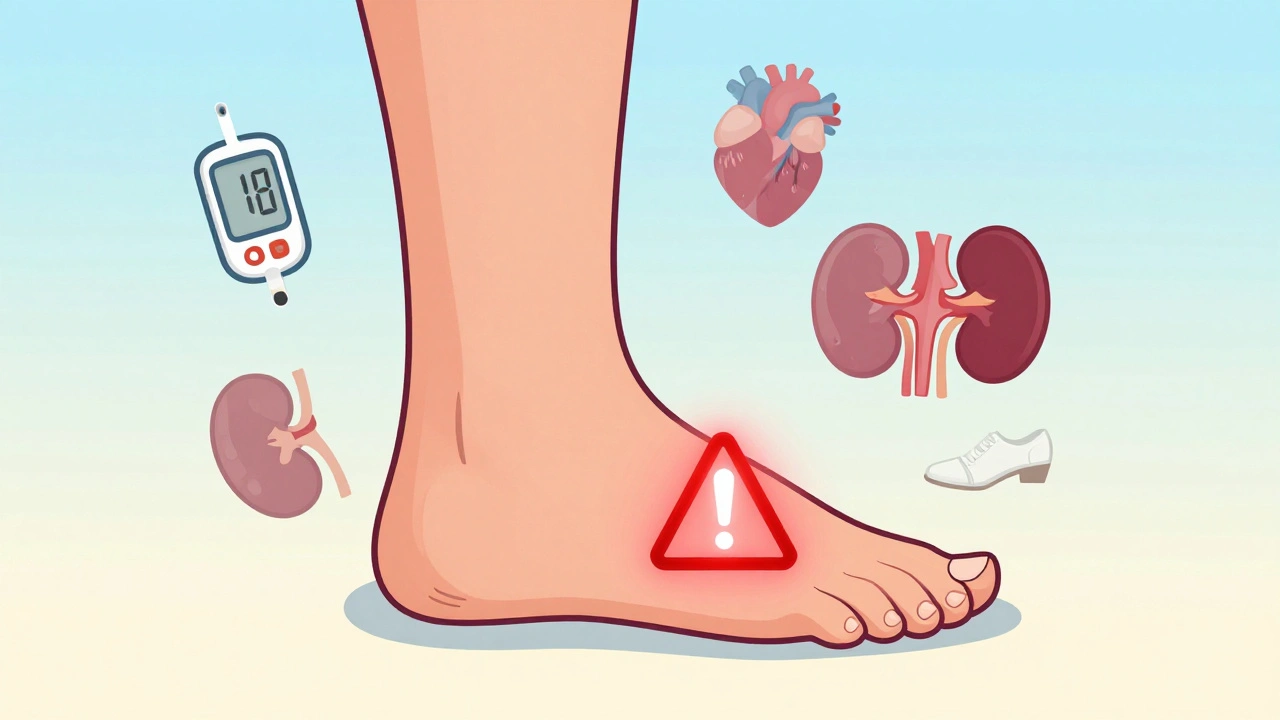
When you’re managing type 2 diabetes, choosing the right medication isn’t just about lowering blood sugar. It’s about balancing benefits with real, sometimes serious, risks. One drug that’s sparked intense debate is canagliflozin-sold under the brand name INVOKANA®. Since its approval in 2013, it’s helped millions of people control their glucose levels and reduce heart failure risk. But it’s also been linked to a higher chance of foot and leg amputations. The question isn’t whether the risk exists-it’s who’s most at risk, and what you can do about it.
What the Data Shows: A Real, But Specific Risk
The alarm bells rang in 2017 after the CANVAS Program, a major study combining two clinical trials, found that people taking canagliflozin had nearly twice the risk of lower-limb amputation compared to those on placebo. The numbers were clear: 5.5 amputations per 1,000 patient-years for the 300 mg dose, versus 2.8 for placebo. That’s not a small difference. It led the FDA to issue a boxed warning-the strongest safety alert they can give. But here’s what’s often missed: that warning was removed in January 2020. Not because the risk disappeared, but because regulators looked deeper. The CREDENCE trial, which focused on patients with diabetic kidney disease, showed that the heart and kidney benefits of canagliflozin outweighed the amputation risk in that group. So the FDA updated the label instead of pulling the drug. Today, the prescribing information still warns about amputation risk-but it’s now in the Warnings and Precautions section, not the boxed warning. The risk is real, but it’s not universal. And it’s not the same across all drugs in the SGLT2 inhibitor class.Why Only Canagliflozin? The Class Difference
It’s easy to assume all SGLT2 inhibitors are the same. They’re not. Empagliflozin (Jardiance) and dapagliflozin (Farxiga) have not shown the same amputation signal in large trials. In fact, dapagliflozin’s DECLARE-TIMI 58 trial showed a trend toward fewer amputations, not more. A 2023 meta-analysis of over 74,000 patients confirmed it: only canagliflozin had a statistically significant increase in amputation risk (odds ratio 1.6). Other SGLT2 inhibitors? No clear link. This isn’t a class effect. It’s specific to canagliflozin. Why? Researchers aren’t 100% sure, but clues point to its stronger effects on blood pressure and weight loss. Canagliflozin lowers systolic blood pressure by about 3.7 mmHg more than other drugs in its class and causes an average weight loss of 2.8 kg. For someone with poor circulation in their legs-common in long-term diabetes-this drop in pressure might reduce blood flow just enough to turn a small sore into a life-altering problem.Who’s Most at Risk?
Not everyone on canagliflozin will face amputation. But some people are far more vulnerable. The highest risk group includes those with:- Pre-existing peripheral artery disease (PAD)-affects 20-30% of people with type 2 diabetes
- Diabetic neuropathy-loss of sensation in the feet, present in about half of long-term patients
- History of foot ulcers or prior amputation
- Current tobacco use
- Absent or weak pedal pulses (a sign of poor circulation)
Real Stories: Patients Speak Up
Behind the statistics are real people. On PatientsLikeMe, nearly 7% of canagliflozin users reported foot problems. Seventeen users specifically mentioned amputation concerns. One Reddit user, u/DiabetesWarrior2020, shared: “After 18 months on Invokana, my podiatrist found a non-healing ulcer. I lost my toe. My endocrinologist switched me to Jardiance right away.” Another, u/SugarFreeLife, said: “Three years on Invokana. No foot issues. My A1c dropped from 8.5% to 6.2%.” Both stories are true. And both matter. The drug works wonders for some. It’s dangerous for others. The difference? Awareness, screening, and early action.What Doctors Should Do-and What You Should Too
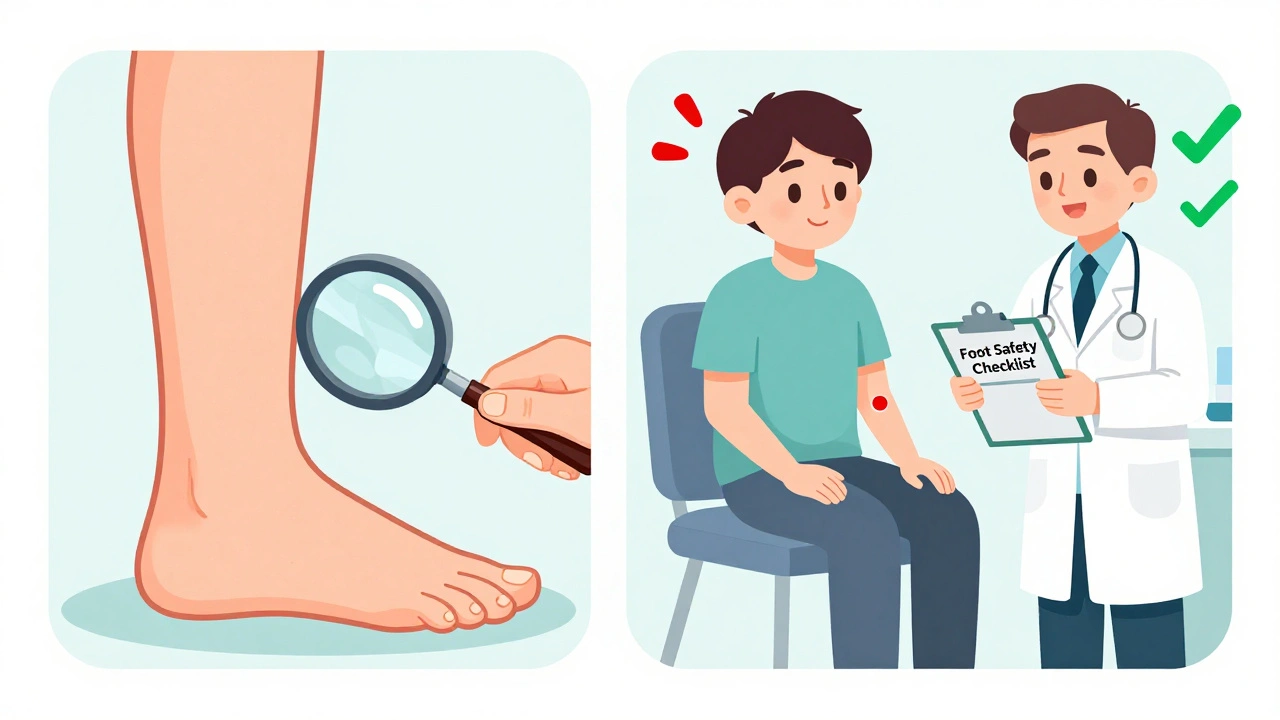
The best way to prevent amputation isn’t to avoid the drug entirely. It’s to use it wisely. Before starting canagliflozin:- Get a full foot exam-check for ulcers, calluses, pulses, and sensation
- Ask for an ankle-brachial index (ABI) test. An ABI under 0.9 means poor leg circulation and is now considered a relative contraindication by the 2025 ADA guidelines
- Review your history: any past foot problems? Smoking? Nerve damage?
- Check your feet daily. Look for redness, swelling, cracks, or sores-even if you don’t feel pain
- Report any new foot pain, warmth, or sores immediately. Don’t wait
- See your podiatrist every 3-6 months if you have any risk factors
- Wear properly fitted shoes. No barefoot walking






11 Comments
This article made me cry. I lost my big toe last year on Invokana. My doctor never asked about my circulation. I thought it was just dry skin. Now I check my feet every night with a mirror. I’m alive because I looked. Please, if you’re on this drug-LOOK. Don’t wait for pain.
It is imperative to underscore that the pharmacological profile of canagliflozin is not monolithic in its risk-benefit calculus across diverse patient populations. The FDA's reclassification of the boxed warning reflects a nuanced, evidence-based recalibration-not an exoneration. For patients with established cardiovascular disease and concomitant renal impairment, the reduction in heart failure hospitalizations and progression of albuminuria remains clinically transformative. However, the absence of a class-wide amputation signal underscores the necessity of precision prescribing. One size does not fit all-especially when microvascular perfusion is compromised.
Okay, let’s get real. I’ve been on Invokana for 4 years and my A1c is 5.9. I’ve got neuropathy, sure-but I check my feet daily, wear diabetic socks, and see my podiatrist every 90 days. My doctor didn’t just hand me a script and say ‘good luck.’ We did the ABI test. My number was 1.1. No red flags. So don’t scare people into stopping a drug that saved their kidneys and heart because of one scary headline. 🙏 Foot care isn’t optional-it’s non-negotiable. And if your doctor isn’t talking to you about it, find a new one. Seriously. Your toes matter.
I’ve seen too many patients on this drug who never had a foot exam. One man came in with gangrene because he thought ‘numb feet’ meant ‘no problem.’ He lost his leg. Canagliflozin isn’t the villain. Negligence is. The system failed him-not the medication. We need mandatory foot screenings before prescribing this. Not suggestions. Requirements.
Y’all. I’m a nurse. I’ve seen the foot ulcers. I’ve held the hands of people who lost toes because they didn’t know how to check. This drug is magic for some. For others? It’s a ticking time bomb. But here’s the thing-you CAN protect yourself. Daily foot checks. No barefoot walking. Socks that don’t squeeze. Shoes that fit. And if your doctor doesn’t mention ABI? Ask for it. Like, right now. You’re worth more than a statistic.
It is critical to note that the amputation risk associated with canagliflozin is statistically significant only in the context of pre-existing peripheral vascular disease and diabetic neuropathy-two conditions that, when co-present, elevate baseline risk regardless of medication. The meta-analysis referenced (2023) explicitly isolates canagliflozin as the sole SGLT2 inhibitor demonstrating this signal, which strongly suggests a pharmacokinetic, not pharmacodynamic, distinction. Moreover, the reduction in major adverse cardiovascular events (MACE) remains robust in high-risk cohorts. Therefore, the appropriate clinical response is not avoidance, but stratification.
Oh please. Another ‘personal story’ from someone who didn’t follow the manual. If you’re diabetic and your feet are numb, you shouldn’t be on ANY SGLT2 inhibitor. Period. The FDA didn’t remove the warning because it’s safe-they removed it because they realized most people are too lazy to get screened. This drug isn’t for you if you can’t be bothered to check your feet. Stop romanticizing ‘personal success stories’-they’re outliers. The data doesn’t lie. And if you’re one of those people who says ‘I’m fine,’ you’re the reason we need mandatory foot exams before prescribing.
Thank you for writing this. I’ve been prescribing canagliflozin for five years. I used to think it was just another SGLT2 inhibitor. Then I had a patient lose two toes. I started doing ABI tests on everyone. Now I don’t even write the script unless the number is above 0.9. It’s not about fear. It’s about responsibility. If you’re on this drug, you deserve a doctor who asks about your feet. If they don’t-ask them to.
so i been on invokana for 3 yrs and no foot issues but my cousin lost a toe and she never checked her feet. like seriously, if you cant check your feet daily dont take this drug. its not the drug its you. also my doc said to get an abi test if you have high bp or smoke. i do both and my abi was 1.0 so im good. just be smart.
The distinction between class effect and drug-specific risk is one of the most important nuances in modern pharmacotherapy. The fact that empagliflozin and dapagliflozin do not carry the same amputation signal-despite identical mechanisms-demonstrates that small differences in molecular structure, renal excretion, or tissue penetration can yield profoundly different clinical outcomes. This is why we cannot generalize drug safety profiles. Canagliflozin’s unique pharmacokinetics, particularly its greater effect on systolic pressure and weight loss, likely contribute to reduced perfusion in vulnerable extremities. This isn’t a failure of the class-it’s a lesson in precision medicine.
My dad’s on Invokana. He’s 72, has PAD, and a 0.85 ABI. His doctor didn’t tell him to stop. I found out by accident. We switched him to Jardiance last month. He’s doing great. No amputation. No drama. Just a better choice. If you’re reading this and your doctor didn’t check your circulation-go back. Bring this article. Ask for the ABI. It takes 5 minutes. It could save your foot.