
When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure it actually does? The answer isn’t in clinical trials on thousands of patients. It’s in a lab, with a machine spinning a basket in a beaker of liquid. That’s dissolution testing - the invisible gatekeeper of generic drug quality.
Why Dissolution Testing Matters
Generic drugs are cheaper because they don’t repeat the expensive clinical trials of brand-name drugs. Instead, they must prove they’re bioequivalent - meaning they release the same amount of active ingredient at the same rate in the body. But testing this in people is slow, expensive, and sometimes unnecessary. That’s where dissolution testing comes in. The FDA uses dissolution testing as a stand-in for human studies. If a generic drug releases its active ingredient the same way as the brand-name version under controlled lab conditions, it’s very likely to behave the same way inside the body. It’s not perfect, but it’s reliable - and it’s the reason you can trust that your $5 generic blood pressure pill works as well as the $100 brand.How the FDA Sets the Rules
The FDA doesn’t guess at dissolution conditions. Every test is built from data. For each drug product, they define exactly:- What kind of machine to use (usually USP Apparatus 1 or 2)
- How fast the basket or paddle spins (typically 50-100 rpm)
- What liquid to use (often pH 1.2 for stomach, pH 6.8 for intestine)
- How much liquid (usually 500-900 mL)
- When to take samples (every 5, 10, 15, 30, 45 minutes)
What Counts as a Pass?
For most immediate-release pills, the rule is simple: at least 80% of the drug must dissolve within 45 minutes. But it’s not that easy. The FDA looks at the full curve - not just one point. Two drugs might hit 80% at 45 minutes, but if one releases 50% at 10 minutes and the other only 20%, they’re not the same. That’s why the FDA uses the f2 similarity factor. It’s a statistical tool that compares the entire dissolution profile of the generic to the brand-name drug. An f2 score of 50 or higher means the two profiles are similar enough to be considered equivalent. A score below 50? The application gets rejected - no matter how clean the clinical data looks.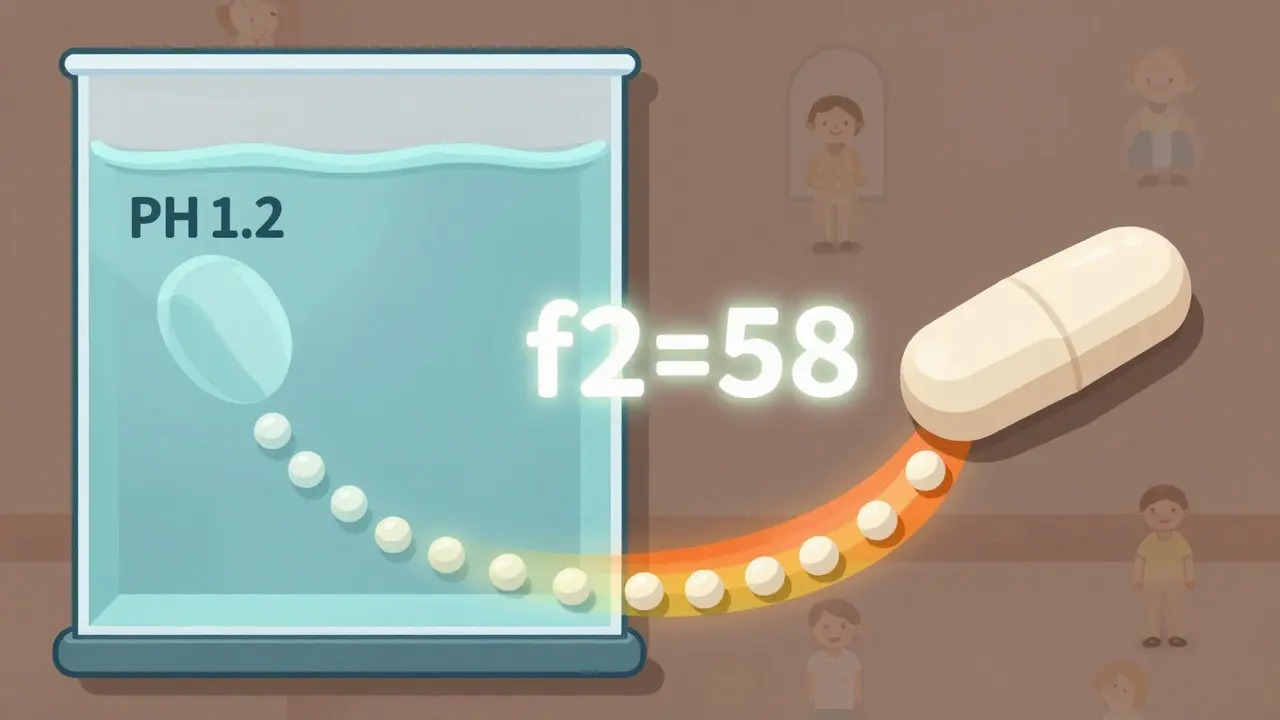
Special Cases: Modified-Release and Low-Solubility Drugs
Not all pills are made the same. Extended-release tablets are designed to release drug slowly over 12 or 24 hours. For these, the FDA requires testing under multiple pH levels - stomach acid, intestinal fluid, and sometimes even with alcohol. Why alcohol? Because if a pill releases all its drug at once when taken with a beer, it could cause overdose. That’s called “dose dumping,” and it’s deadly. Low-solubility drugs - like some antifungals or cholesterol meds - are even trickier. These drugs don’t dissolve easily, so their release depends heavily on the formulation. The FDA requires extra proof that the dissolution method can tell the difference between a good and bad version. A method that can’t detect a faulty formula is useless. That’s why manufacturers often spend 6-12 months just developing the right test.BCS Class I: When You Can Skip Human Studies
Some drugs are so well-behaved that the FDA lets manufacturers skip human bioequivalence studies entirely. These are drugs classified as BCS Class I: highly soluble and highly permeable. Examples include metformin, atenolol, and ciprofloxacin. For these, the FDA says: if your generic dissolves at least 85% in 30 minutes using 900 mL of 0.1N HCl, you’re done. No blood draws. No volunteers. Just a lab test. This is called a “biowaiver.” It saves millions of dollars and speeds up access to affordable medicines. As of 2023, over 1,200 drug products qualify for this shortcut.The FDA’s Dissolution Database
Manufacturers don’t start from scratch. The FDA maintains a public Dissolution Methods Database with recommended test methods for over 2,800 drug products. If your generic is on the list, you follow the method. If it’s not, you have to develop your own - and prove it works. This database is updated constantly. It’s the single most used resource by generic drug companies. Without it, every new generic would require years of trial and error. With it, approval timelines shrink from years to months.
What Happens After Approval?
Approval isn’t the end. The FDA’s SUPAC-IR guidelines require manufacturers to prove that any change - new factory, different supplier, even a tweak in tablet coating - doesn’t alter the dissolution profile. If it does, they must resubmit data. That’s why you won’t see sudden changes in how your generic pill looks or tastes. The FDA checks it. Manufacturers submit up to 100 pages of dissolution data in their ANDA applications. Reviewers pore over every curve, every time point, every statistical calculation. One mismatched f2 score can delay approval for months.Why This System Works
Dissolution testing isn’t just about rules. It’s about science. It’s a bridge between what happens in a test tube and what happens in your bloodstream. The FDA doesn’t trust assumptions. They demand evidence - and they’ve built a system that forces manufacturers to prove equivalence before a single pill leaves the factory. It’s why generic drugs in the U.S. are among the safest in the world. It’s why millions of people can afford their prescriptions. And it’s why you can trust that the little white pill you take every morning - no matter the brand - will do exactly what it’s supposed to.What’s Next?
The FDA is exploring ways to make dissolution testing even smarter. One idea: using fluids that mimic the actual conditions inside the gut - not just pH, but enzymes, bile salts, and food effects. Another: expanding biowaivers to BCS Class III drugs (high solubility, low permeability). If approved, this could cut development time for dozens of new generics. By 2025, experts predict 35% of generic approvals will rely on standardized dissolution methods - up from 25% in 2020. The goal isn’t to make testing easier. It’s to make it more predictive. Better science. Faster access. Lower cost. That’s the FDA’s quiet promise to every patient who needs medicine.What is dissolution testing in generic drugs?
Dissolution testing is a lab procedure that measures how quickly a drug releases its active ingredient from a tablet or capsule under controlled conditions. The FDA uses it to prove that a generic drug releases the drug at the same rate and amount as the brand-name version, ensuring they work the same way in the body without needing human studies.
How does the FDA decide if a generic drug passes dissolution testing?
The FDA compares the dissolution profile of the generic drug to the brand-name version using a statistical measure called the f2 similarity factor. A score of 50 or higher means the two profiles are similar enough to be considered equivalent. For immediate-release pills, at least 80% of the drug must dissolve within 45 minutes under specified conditions.
Do all generic drugs need dissolution testing?
Yes - all oral solid dosage forms (tablets, capsules) and oral suspensions require dissolution testing. Liquid drugs, topical creams, and injectables are exempt because they’re already dissolved or absorbed differently. The FDA requires it for all products where release rate affects safety and effectiveness.
What is a biowaiver, and how does dissolution testing relate to it?
A biowaiver lets manufacturers skip human bioequivalence studies if their drug meets certain criteria. For BCS Class I drugs (high solubility, high permeability), if the generic dissolves at least 85% in 30 minutes under standardized conditions, the FDA accepts it as bioequivalent without testing in people. Dissolution testing is the key to qualifying for this shortcut.
Why does the FDA require dissolution testing for alcohol exposure?
Some extended-release pills can release their entire dose at once if taken with alcohol - a dangerous effect called dose dumping. To prevent this, the FDA requires manufacturers to test their products in solutions containing up to 40% ethanol. If the drug releases too quickly under these conditions, the product is rejected or requires a warning label.
Can a generic drug pass bioequivalence but fail dissolution testing?
It’s rare, but it happens. Sometimes, a generic may show acceptable blood levels in human studies but have a different dissolution profile than the brand. In those cases, the FDA may approve the drug but set different dissolution specifications - meaning the generic’s release curve is accepted as equivalent even if it doesn’t match the brand exactly. This is documented in approved ANDAs and reflects real-world variability.
How often does the FDA update its dissolution testing guidelines?
The FDA updates its guidance documents regularly, often in response to new science or industry feedback. The latest major update was in September 2023, which clarified that all dissolution methods - whether from USP, FDA, or developed internally - must be validated for product-specific performance. The Dissolution Methods Database is also updated monthly with new test methods for over 2,800 drugs.






11 Comments
Man, I never thought about how much science goes into those little white pills I swallow every morning 🤯
It’s wild that a spinning basket in a beaker is what keeps me from overdosing on my blood pressure med.
Like... I just assume it works. But the FDA’s actually doing this intricate dance between chemistry and public safety.
And the f2 factor? That’s some next-level stats magic.
They’re not just guessing - they’re mapping release curves like astronomers tracking stars.
It’s comforting, honestly. In a world full of snake oil, this is real science.
Also, the alcohol thing? Yeah, I’ve taken my ER pill with a beer before. Thanks for the wake-up call.
Maybe I’ll switch to tea.
Also also - biowaivers for BCS Class I? That’s genius. Saves money, saves time, saves lives.
Generic drugs aren’t cheap because they’re bad. They’re cheap because the system’s smart.
Shoutout to the lab techs in white coats spinning baskets all day.
We don’t see them, but they’re the real MVPs.
Also, can we make a subreddit for dissolution testing? r/DissolutionGang?
I’d subscribe.
Also also also - I’m gonna start telling my grandma this next time she says generics are ‘fake medicine’.
So the pill I take for anxiety is basically being tested with fake stomach juice? 😮
Wow. So we’re trusting a machine that spins in water to decide if your life-saving drug works?
Not a single human trial? Just… math?
And you call this medicine?
It’s like letting a toaster pass a fire safety test by checking if it’s warm.
What’s next? Testing insulin with a ruler?
At least back in the day, we had doctors who knew what they were doing.
Now it’s all spreadsheets and robots.
Scary.
And don’t even get me started on the ‘biowaiver’ nonsense.
You’re telling me we skip human testing for drugs that go into people’s bodies?
That’s not science.
That’s corporate laziness dressed up as efficiency.
And the FDA? They’re not protecting us.
They’re just rubber-stamping for Big Pharma.
Wake up, people.
Dissolution profiling via USP Apparatus II under sink conditions is non-negotiable for BCS Class I compounds
Any deviation from f2 >50 constitutes non-equivalence regardless of Cmax or AUC
Biowaiver eligibility requires dissolution ≥85% in 30min at pH 1.2
Manufacturers often fail due to excipient variability
Supac-IR mandates post-approval changes to be validated via dissolution kinetics
Database is underutilized by emerging markets
India lacks regulatory alignment
Generic manufacturers here still rely on bioequivalence studies
Wasteful
Inefficient
Outdated
I love how this post breaks down something so technical into something anyone can understand.
It’s easy to think generics are ‘lesser’ - but this shows they’re actually held to a really high bar.
And the fact that the FDA tests for alcohol interactions? That’s next-level patient safety.
I’ve got a friend on extended-release Adderall - I’m gonna send them this.
Also, the dissolution database being public? That’s transparency we don’t always get in pharma.
It’s rare to see a government agency this thoughtful.
Thanks for sharing this.
It made me appreciate my meds a little more.
And honestly? That’s rare.
ok so i just learned that my generic omeprazole is being tested in fake stomach acid???
and also like if you drink alcohol with it it might explode???
wait no not explode but like release all the drug at once???
that’s wild
i always thought generics were just copied pills
turns out they’re like… scientifically engineered replicas
and the f2 thing??
imagine having to compare entire curves instead of just one number
so cool
also i just took mine with a beer
oops
but i’m gonna be fine right??
right??
also why does this feel like a spy movie??
the FDA is out here with their dissolution labs like secret agents
love it
So let me get this straight - you’re telling me the FDA lets companies skip human trials and just rely on a spinning basket?
And you call that ‘science’?
What if the company fakes the data?
What if the machine breaks?
What if the pH is off by 0.1?
One tiny error and someone dies.
And you’re okay with that?
People are dying because of this.
It’s not ‘safe’ - it’s a gamble.
And the FDA is just sitting there with their spreadsheets while real people suffer.
Why don’t they test on real humans?
Why is profit more important than safety?
Wake up.
This isn’t innovation.
This is negligence.
And you’re defending it?
Pathetic.
THIS IS WHY AMERICA STILL LEADS IN PHARMA
NOBODY ELSE HAS THIS LEVEL OF RIGOR
EU? THEY’RE STILL USING 1990s METHODS.
INDIA? THEY’RE CUTTING CORNERS TO SAVE A BUCK.
CHINA? DON’T EVEN ASK.
THE FDA IS THE ONLY ONE THAT GIVES A DAMN
AND YET WE HAVE PEOPLE LIKE ASTRID AND APRIL COMPLAINING ABOUT ‘ROBOTS’?
YOU WANT TO LIVE IN A COUNTRY WHERE YOUR GENERIC MEDS ARE A ROLL OF THE DICE?
NO THANKS.
THESE LAB TECHS ARE THE REAL HEROES
THEY’RE NOT JUST SPINNING BASKETS - THEY’RE SPINNING LIVES
IF YOU’RE ON A GENERIC AND YOU’RE STILL BREATHING - THANK A DISSOLUTION SCIENTIST
AND STOP WHINING.
WE HAVE THE BEST SYSTEM IN THE WORLD.
USE IT.
APPRECIATE IT.
AND FOR ONCE - DON’T BE A NAYSAYER.
Yo, this whole thing is like a Bollywood movie where the hero’s identity is hidden until the climax - and the hero? It’s the dissolution curve.
Who knew a simple test could be the difference between life and death?
And the f2 score? That’s the plot twist.
You think you’re just taking a pill - but behind it? A whole symphony of science.
It’s not just chemistry.
It’s poetry.
Every timepoint is a verse.
Every pH change, a chord.
And the FDA? They’re the conductor.
They don’t just allow generics - they demand perfection.
And yet we call them ‘bureaucrats’?
No.
They’re the quiet guardians of our health.
So next time you pop a generic - pause.
And say thank you.
To the basket.
To the beaker.
To the data.
To the unseen hands.
They’re the reason you’re still here.
And that’s more than just medicine.
That’s magic.
The claim that dissolution testing is a reliable proxy for bioequivalence is scientifically overstated. In vitro-in vivo correlation (IVIVC) is only established for a subset of drugs, and even then, it is highly formulation-dependent. The f2 similarity factor is a statistical convenience, not a biological validation. Regulatory reliance on dissolution profiles without IVIVC validation represents a significant gap in pharmacokinetic assurance. Furthermore, the dissolution database contains methods that are not always validated for product-specific variability. This system works for Class I drugs, but extrapolating it to all generics is methodologically unsound. The FDA's approach, while pragmatic, is not scientifically rigorous - it is regulatory expediency disguised as science. Human bioequivalence studies remain the gold standard for a reason.
@Andrew Clausen - I get your point. IVIVC isn’t perfect.
But you’re ignoring the scale.
We’re talking about over 1,200 biowaivers approved since 2010.
Zero major safety events linked to dissolution-based approvals.
Meanwhile, every single human bioequivalence study has a 10-20% failure rate due to inter-subject variability.
So we’re choosing between a lab test that’s been validated across thousands of batches…
or a human trial where one person’s gut pH changes the outcome?
Which one’s actually more reliable?
And yes, the FDA updates methods constantly.
They don’t just slap a test on a drug and call it done.
They iterate.
They validate.
They retest.
They publish.
That’s not expediency.
That’s adaptive science.
And if you’re still worried?
Then take the brand-name.
But don’t tell the rest of us we’re unsafe because we can’t afford it.
That’s not science.
That’s privilege.